Accuracy of measurements with the help of so-called measuring instruments is constantly increasing with the growth of science (Measurements; Units of measures - absolute systems). It now depends not only on the careful preparation of instruments, but also on the discovery of new measurement principles. Thus, for example, the colors of thin plates - a phenomenon of light interference - make it possible to measure linear quantities much smaller than the most precise screw micrometers. The bolometer measures thermal changes in many cases much smaller than those available to the thermomultiplier. However, a general remark can be made that new measurement methods much more often lead to an increase in the accuracy of determinations very small changes one or another value than to increase the accuracy of determination this whole value.
Encyclopedic Dictionary F.A. Brockhaus and I.A. Efron. - St. Petersburg: Brockhaus-Efron. 1890-1907 .
See what “Measurement accuracy” is in other dictionaries:
Accuracy of measurements- Quality of measurements, reflecting the closeness of their results to the true value of the measured value Source: GOST 24846 81: Soils. Methods for measuring deformations of the foundations of buildings and structures...
A characteristic of the quality of measurements, reflecting the degree of closeness of the measurement results to the true value of the measured quantity. The smaller the measurement result deviates from the true value of the quantity, i.e., the smaller its error, the higher T... Physical encyclopedia
accuracy of measurements- - [L.G. Sumenko. English-Russian dictionary of information technologies. M.: State Enterprise TsNIIS, 2003.] Topics information technology in general EN accuracy of measurements ...
accuracy of measurements- verification. believe. The device is lying. see show time... Ideographic Dictionary of the Russian Language
GOST R EN 306-2011: Heat exchangers. Measurements and measurement accuracy when determining power- Terminology GOST R EN 306 2011: Heat exchangers. Measurements and measurement accuracy when determining power: 3.31 impact magnitude: A quantity that is not the subject of measurement, but can influence the result obtained. Definitions of the term from... ... Dictionary-reference book of terms of normative and technical documentation
measurement result accuracy- measurement accuracy One of the characteristics of measurement quality, reflecting the proximity to zero error of the measurement result. Note. It is believed that the smaller the measurement error, the greater its accuracy. [RMG 29 99] Topics: metrology,... ... Technical Translator's Guide
accuracy- 3.1.1 accuracy: The degree of closeness of a measurement result to an accepted reference value. Note The term "accuracy", when referring to a series of measurement results, includes a combination of random components and an overall systematic... ... Dictionary-reference book of terms of normative and technical documentation
Measuring instruments The degree of agreement between the readings of a measuring device and the true value of the measured quantity. The smaller the difference, the greater the accuracy of the device. The accuracy of a standard or measure is characterized by an error or degree ... ... Wikipedia
accuracy- The degree of closeness of the measurement result to the accepted reference value. Note. The term "accuracy", when it refers to a series of measurement results (tests), includes a combination of random components and an overall systematic... ... Technical Translator's Guide
measuring instrument accuracy- accuracy A characteristic of the quality of a measuring instrument, reflecting the proximity of its error to zero. Note. It is believed that the smaller the error, the more accurate the measuring instrument. [RMG 29 99] Topics metrology, basic concepts Synonyms accuracy ... Technical Translator's Guide
Books
- Physical foundations of measurements in technology. food and chemical industries. Textbook, Popov Gennady Vasilievich, Zemskov Yuri Petrovich, Kvashnin Boris Nikolaevich Series: Textbooks for universities. Special literature Publisher: Lan,
- Physical foundations of measurements in food and chemical industry technologies. Textbook, Popov Gennady Vasilievich, Zemskov Yuri Petrovich, Kvashnin Boris Nikolaevich, This manual provides brief theoretical information about the laws of measurements, measuring systems, elements of the physical picture of the world, as well as the principles of measurements based on ... Series: Textbooks for universities. Special literature Publisher:
The methods used in measurement differ in the equipment used, the complexity or simplicity of the measurements and, accordingly, the metrological characteristics of the result obtained, mainly accuracy.
Method of measurement is a set of methods and techniques for comparing the measured PV with its unit in accordance with the implemented measurement principle.
Measuring principle is a physical phenomenon or effect that forms the basis of measurements. For example, measuring mass on a scale (using gravity)
Measurement result– this is the value of the measured quantity obtained by taking measurements.
Measurement result error– deviation of the measurement result from the true (actual) value of the measured quantity
Accuracy of measurements– one of the characteristics of measurement quality, reflecting the proximity to 0 error of the measurement result.
Credibility is a characteristic of the quality of measurements, reflecting the degree of confidence in their results and the confidence probability that the true value of the measured value is within the specified confidence limits.
Convergence measurement results– closeness to each other of the results of measurements of the same quantity, carried out under the same conditions using the same method.
Reproducibility– characteristic of measurement quality, reflecting the closeness to each other of measurement results obtained in different places, by different methods, by different means, by different operators at different times, but reduced to the same conditions (temperature, pressure, humidity and the level of existing interference - EM -interference, ES interference, optical)
Correctness of the measurement result– characteristic of the quality of measurements, reflecting the closeness to 0 of the systematic error (an error that occurs in all measurements of a quantity; the magnitude of the error can be established and therefore eliminated).
When carrying out measurements it is assumed:
1.comparison measured PV with a homogeneous PV taken as a unit (a comparator is used)
2.measurement conversion– converting an input quantity into an output quantity using a known connection between these quantities. The output signals of the measuring converters are unified: voltage 0..10 and DC current 0..5mA, 4..20mA, 0..20mA
3.scaling– formation of an output signal homogeneous with the input signal, the size of the informative parameter of the output signal is proportional to the size of the informative parameter of the input signal (implemented using a scale converter)
Measurement methods are classified according to various criteria:
The physical principle underlying the measurement (electrical, mechanical, magnetic, optical measurements)
The degree of interaction between the means and the object of measurement - contact and non-contact (measurement of temperature of resistance thermometers, measurement of temperature of pyrometers by radiation)
Mode of interaction between the means and the measurement object (static and dynamic)
Type of measuring signals (analog and digital)
Organization of comparison of the measured value with a measure (direct assessment / counting method - a method in which the value of the measured value is determined directly using an indicating measuring instrument - is simple, but the accuracy is low. Comparison with a measure method - the measured value is compared with the value reproduced by the measure - these methods difficult to implement, but characterized by high accuracy, divided into differential, zero, substitution, coincidence)
Differential (difference method) - the measuring device evaluates the difference between the measured quantity and a homogeneous quantity having a known value. The accuracy of the method increases as the difference between the compared values decreases.
The zero method is a special case of the differential method, and consists in the fact that the resulting effect of the influence of the measured quantity and the reference measure on the comparison device is brought to 0 (measurement of electrical resistance using a bridge circuit with full balancing of the bridge by adjusting the ratings of its elements).
Substitution method - the measured quantity is replaced by a measure with a known value of the quantity
Coincidence method - measure the difference between the desired value and the reference measure using coincidences of marks or periodic signals (when measuring displacement, period, frequency).
The application of the elements of the general theory of measurements discussed above is necessary to ensure the accuracy and reliability of the measurement result. With repeated observations, a number of values are obtained, which are processed to find the measurement result. For processing, mathematical statistics tools are used, considering a series of values as a sample from the general population. Based on probability theory, mathematical statistics allows one to assess the reliability and accuracy of conclusions made on the basis of limited statistical material.
Accuracy characterized by the inverse value relative error. Reciprocal value absolute error, is called a measure of accuracy. Depending on the required accuracy, both single and multiple observations can be used in the measurement process. If only one observation is made, then the result of the observation is the result of the measurement. If more than one observation is performed, the measurement result is obtained as a result of processing the observation results, usually in the form of an arithmetic mean.
The required accuracy of technical measurements can also be ensured by repeating multiple observations. In this case, multiple observations of the same object are performed several times. To reduce the time required to process several series of multiple observations, indicators are used at the beginning of the processing process to determine the preferred series and subsequently process only this series.
Such indicators are the sum of residual errors and the sum of squares of residual errors. These indicators are an indirect characteristic of the unbiasedness and efficiency of the estimate obtained by processing the results of multiple observations.
If measurements were carried out several times and several series of observation results were obtained, then with the same number of observations in different series, the series in which the results were distributed symmetrically relative to the arithmetic mean value will have the smallest sum of residual errors, i.e. closest to the normal law. For further calculations, it is recommended to choose this one, because it will satisfy to the greatest extent the condition of equiprecision, and with the systematic error excluded, the condition of unbiased assessment of the measurement result.
Unbiased Estimate - Statistical estimate, mathematical expectation which coincides with the estimated value. An unbiased estimate is said to be free from systematic error.
However, symmetry is not an exhaustive characteristic of the distribution. The next important feature in metrology is the compactness of the distribution. Based on this criterion, with a fixed number of observations, the preferred series can be determined by an efficiency indicator. The effective one is the one of several possible unbiased estimates that has the smallest variance. The efficiency condition will be satisfied by the series with the least sum of squared residual errors.
Obviously, in practical metrology, efficient estimation is preferable. The sign of effectiveness indicates that the subjective component of the random error is minimal, the observations were carried out more accurately and the smallest amount of random error will be ensured.
In theoretical metrology, a consistent assessment is also considered, which is ideal model for multiple measurements, which is desirable to strive for, but almost impossible to achieve. With a consistent estimate, the true and actual value coincide, the error is zero. This is achieved by an infinite increase in the number of observations. A consistent estimate is one in which, as the number of observations tends to infinity, the variance tends to zero.
Credibility the measurement result is considered high if it is close to unity (is the probability with which the true value of a physical quantity is removed from the actual value by an interval not exceeding the error). In technical measurements, the value is usually taken equal to 0.95. This suggests that if such measurements are carried out 100 times, then in 95 cases the true value will be removed from the actual value by an interval whose dimensions do not exceed the error, and in 5 cases it will be removed by an interval exceeding the error. Therefore, in measurements that have a direct impact on safety and health, the value is assumed to be 0.99. The same probability is assigned for single measurements. This is explained by the fact that, other things being equal (primarily, with the same number of observations), the sizes and are interrelated: the larger , the more , therefore, by assigning a high degree of confidence, we are considering the worst case of controlled events.
By assigning a greater degree of uncertainty to measured events, we gain greater confidence that they will occur.
There is a way to simultaneously increase the reliability and reduce the uncertainty of the measurement result, i.e. increase and decrease. This method is to increase the number of observations. However, additional observations make the measurement process more expensive. In this regard, the issue of correct recording of measurement results, discussed in the first section, is relevant.
2.5. Direct equal-precision measurements with multiple observations
The method of direct equal-precision measurements with multiple observations is fundamental; it is used in technical measurements to increase the reliability of the result; it is the basis for many metrological measurement methods and for indirect measurement methods.
The classification of direct and multiple measurements is discussed above. The requirement for direct measurements is related to the rules for taking into account errors. Modern measuring instruments, as a rule, are complex devices that perform indirect measurement of physical quantities. However, the results are usually considered as the results of direct measurements, since the error of indirect measurements within the measuring instrument is already taken into account by its accuracy class.
Equal accuracy of measurements is interpreted in a broad sense as identical distribution (in a narrow sense, equal accuracy of measurements is understood as the same measure of accuracy of all measurement results). The presence of gross errors (misses) means a violation of equivalence in both the broad and narrow sense.
In practice, the condition of equal accuracy is considered satisfied if observations are made by the same operator, under the same environmental conditions, using the same measuring instrument. Under such conditions, equally scattered (in other words, equally accurate, from the words equal accuracy) will be obtained, i.e. identically distributed random variables
The method of direct equal-precision measurements with multiple observations is set out in GOST 8.207 - 76. In this section, in addition to GOST 8.207 - 76, the information and comments necessary to perform the calculations are provided.
Comments to GOST 8.207 - 76. Section 2. Measurement result and estimate of its standard deviation
The measurement result is found as the arithmetic mean of the observation results:

where is the number of observations.
1. Subject and tasks of metrology
Metrology means the science of measurements, existing means and methods that help to maintain the principle of their unity, as well as ways to achieve the required accuracy.
The origin of the term “metrology” itself is traced back to two Greek words: metron, which translates as “measure,” and logos, “teaching.” The rapid development of metrology occurred at the end of the twentieth century. It is inextricably linked with the development of new technologies. Before this, metrology was only a descriptive scientific subject. Thus, we can say that metrology studies:
1) methods and means for accounting for products according to the following indicators: length, weight, volume, consumption and power;
2) measurements of physical quantities and technical parameters, as well as the properties and composition of substances;
3) measurements for monitoring and regulation of technological processes.
There are several main areas of metrology:
1) general measurement theory;
2) systems of units of physical quantities;
3) methods and means of measurement;
4) methods for determining measurement accuracy;
5) the basis for ensuring the uniformity of measurements, as well as the basis for the uniformity of measuring instruments;
6) standards and exemplary measuring instruments;
7) methods for transferring unit sizes from samples of measuring instruments and from standards to working measuring instruments.
It is also necessary to distinguish between the objects of metrology: 1) units of measurement of quantities;
2) measuring instruments;
3) techniques used to perform measurements, etc.
Metrology includes: firstly, general rules, norms and requirements, and secondly, issues that require state regulation and control. And here we are talking about:
1) physical quantities, their units, as well as their measurements;
2) principles and methods of measurements and measuring equipment;
3) errors of measuring instruments, methods and means of processing measurement results in order to eliminate errors;
4) ensuring the uniformity of measurements, standards, samples;
5) state metrological service;
6) methodology of verification schemes;
7) working measuring instruments.
In this regard, the tasks of metrology become: improvement of standards, development of new methods of precise measurements, ensuring the unity and necessary accuracy of measurements.
2 Classification of measurements
Classification of measuring instruments can be carried out according to the following criteria.
1. Accuracy characteristics measurements are divided into equal and unequal.
Equal-precision measurements a physical quantity is a series of measurements of a certain quantity made using measuring instruments (MI) with the same accuracy under identical initial conditions.
Unequally accurate measurements a physical quantity is a series of measurements of a certain quantity made using measuring instruments with different accuracy and (or) under different initial conditions.
2. By number of measurements measurements are divided into single and multiple.
3. By type of change in value measurements are divided into static and dynamic.
Static measurements- These are measurements of a constant, unchanging physical quantity.
Dynamic measurements– these are measurements of a changing, non-constant physical quantity.
4. By purpose measurements are divided into technical and metrological.
Technical measurements– these are measurements performed by technical measuring instruments.
Metrological measurements are measurements made using standards.
5. By way of presenting the result measurements are divided into absolute and relative.
Absolute measurements– these are measurements that are performed through direct, direct measurement of a fundamental quantity and (or) the application of a physical constant. Relative measurements– these are measurements in which the ratio of homogeneous quantities is calculated, with the numerator being the quantity being compared, and the denominator being the basis of comparison (unit).
6. By methods of obtaining results measurements are divided into direct, indirect, cumulative and joint.
Direct measurements– these are measurements performed using measures, i.e. the measured quantity is compared directly with its measure. An example of direct measurements is the measurement of an angle (measure - protractor).
Indirect measurements are measurements in which the value of the measured quantity is calculated using values obtained through direct measurements.
Aggregate Measurements– these are measurements, the result of which is the solution of a certain system of equations. Joint measurements– these are measurements during which at least two inhomogeneous physical quantities are measured in order to establish the relationship that exists between them.
3. Basic measurement characteristics
The following main measurement characteristics are distinguished:
1) the method by which measurements are taken;
2) measurement principle;
3) measurement error;
4) measurement accuracy;
5) correctness of measurements;
6) reliability of measurements.
Measurement method- this is a method or a set of methods by which a given quantity is measured, i.e., a comparison of the measured quantity with its measure according to the accepted principle of measurement.
There are several criteria for classifying measurement methods.
1. According to the methods of obtaining the desired value of the measured quantity, the following are distinguished:
1) direct method (carried out using direct, direct measurements);
2) indirect method.
2. According to measurement techniques, there are:
1) contact measurement method;
2) non-contact measurement method.
Contact measurement method based on direct contact of any part of the measuring device with the measured object.
At non-contact measurement method The measuring device does not come into direct contact with the object being measured.
3. According to the methods of comparing a quantity with its measure, the following are distinguished:
1) direct assessment method;
2) method of comparison with its unit.
Direct assessment method is based on the use of a measuring device that shows the value of the measured quantity.
Method of comparison with measure based on comparing the object of measurement with its measure.
Measuring principle– this is a certain physical phenomenon or their complex on which the measurement is based.
Measurement error is the difference between the result of measuring a quantity and the real (actual) value of this quantity.
Accuracy of measurements– this is a characteristic that expresses the degree of correspondence of the measurement results to the real value of the measured quantity.
Correct measurement– this is a qualitative characteristic of a measurement, which is determined by how close to zero the value of a constant or fixed error that changes during repeated measurements (systematic error).
Reliability of measurements is a characteristic that determines the degree of confidence in the obtained measurement results.
4 The concept of physical quantity The meaning of systems of physical units
A physical quantity is a concept of at least two sciences: physics and metrology. By definition, a physical quantity is a certain property of an object or process, common to a number of objects in terms of qualitative parameters, but differing, however, in quantitative terms (individual for each object). There are a number of classifications created according to various criteria. The main ones are divided into:
1) active and passive physical quantities – when divided in relation to measurement information signals. Moreover, the first (active) in this case are quantities that, without the use of auxiliary energy sources, have the probability of being converted into a measurement information signal. And the second (passive) are quantities for which it is necessary to use auxiliary energy sources that create a signal of measurement information;
2) additive (or extensive) and non-additive (or intensive) physical quantities - when dividing on the basis of additivity. It is believed that the first (additive) quantities are measured in parts; in addition, they can be accurately reproduced using a multivalued measure based on the summation of the sizes of individual measures. But the second (non-additive) quantities are not directly measured, since they are converted into a direct measurement of a quantity or a measurement by indirect measurements. In 1791, the first ever system of units of physical quantities was adopted by the French National Assembly. It was a metric system of measures. It included: units of length, area, volume, capacity and weight. And they were based on two now well-known units: the meter and the kilogram.
The scientist based his methodology on three main independent quantities: mass, length, time. And the mathematician took the milligram, millimeter and second as the main units of measurement for these quantities, since all other units of measurement can be easily calculated using the minimum ones. Thus, at the present stage of development, the following main systems of units of physical quantities are distinguished:
1) GHS system(1881);
2) MKGSS system(end of the 19th century);
3) MKSA system(1901)
5. International system of units
The decisions of the General Conference on Weights and Measures adopted the following definitions of the basic units of measurement of physical quantities:
1) a meter is considered the long path that light travels in a vacuum in 1/299,792,458 of a second;
2) the kilogram is considered equivalent to the existing international prototype of the kilogram;
3) a second is equal to 919,2631,770 periods of radiation corresponding to the transition that occurs between two so-called hyperfine levels of the ground state of the Cs133 atom;
4) the ampere is considered a measure of the strength of a constant current that causes an interaction force on each section of a conductor 1 m long, provided that it passes through two straight parallel conductors having such indicators as a negligible circular cross-sectional area and an infinite length, as well as being located at a distance of 1 m from each other in vacuum conditions;
5) a kelvin is equal to 1/273.16 of the thermodynamic temperature, the so-called triple point of water;
6) a mole is equal to the amount of substance of the system, which includes the same number of structural elements as atoms in C 12 weighing 0.01 2 kg.
In addition, the International System of Units contains two quite important additional units needed to measure plane and solid angles. So, the unit of a plane angle is the radian, or rad for short, which is the angle between two radii of a circle, the length of the arc between which is equal to the radius of the circle. If we are talking about degrees, then the radian is equal to 57 ° 17 "48". And the steradian, or sr, taken as a unit of solid angle, is, accordingly, a solid angle, the location of the vertex of which is fixed in the center of the sphere, and the area cut out by this angle on the surface of the sphere, is equal to the area of a square whose side is equal to the length of the radius of the sphere. Other additional SI units are used to form units of angular velocity, as well as angular acceleration, etc. The radian and steradian are used for theoretical constructions and calculations, since most of the significant ones. For practice, the values of angles in radians are expressed by transcendental numbers. Non-systemic units include the following:
1) a tenth of a white, decibel (dB), is taken as a logarithmic unit;
2) diopter - luminous intensity for optical instruments;
3) reactive power – Var (VA);
4) astronomical unit (AU) – 149.6 million km;
5) light year, which refers to the distance that a ray of light travels in 1 year;
6) capacity – liter;
7) area – hectare (ha).
There are also units that are not included in the SI at all. These are primarily units such as degrees and minutes. All other units are considered derivatives, which, according to the International System of Units, are formed using the simplest equations using quantities whose numerical coefficients are equal to one. If the numerical coefficient in an equation is equal to one, the derived unit is called coherent.
6. Physical quantities and measurements
The object of measurement for metrology, as a rule, is physical quantities. Physical quantities are used to characterize various objects, phenomena and processes. Separate basic and derivative quantities from basic quantities. Seven basic and two additional physical quantities are established in the International System of Units. These are length, mass, time, thermodynamic temperature, amount of matter, luminous intensity and electric current, additional units are radian and steradian. Physical quantities have qualitative and quantitative characteristics.
The qualitative difference in physical quantities is reflected in their dimensions. The dimension designation is established by the international ISO standard; it is the symbol dim*.
The quantitative characteristic of a measured object is its size obtained as a result of measurement. The most basic way to obtain information about the size of a certain quantity of a measurement object is to compare it with another object. The result of such a comparison will not be an exact quantitative characteristic; it will only make it possible to find out which of the objects is larger (smaller) in size. Not only two, but also a larger number of sizes can be compared. If the sizes of measurement objects are arranged in ascending or descending order, you will get order scale. The process of sorting and arranging sizes in ascending or descending order on an order scale is called ranking. For the convenience of measurements, certain points on the order scale are fixed and called reference or reference points. Fixed points on the order scale can be assigned numbers, which are often called points.
Reference order scales have a significant drawback: the uncertain value of the intervals between fixed reference points.
The best option is the ratio scale. A ratio scale is, for example, the Kelvin temperature scale. On this scale there is a fixed reference point - absolute zero (the temperature at which the thermal movement of molecules stops). The main advantage of the ratio scale is that it can be used to determine how many times one size is larger or smaller than another.
The size of a measurement object can be represented in different forms. This depends on the intervals into which the scale used to measure a given size is divided.
For example, travel time can be presented in the following forms: T = 1 hour = 60 min = 3600 s. These are the values of the measured quantity. 1, 60, 3600 are the numerical values of this value.
7. Standards and reference measuring instruments
All issues related to the protection, use and creation of standards, as well as monitoring their condition, are resolved according to the uniform rules established by GOST “GSI. Standards of units of physical quantities. Basic provisions" and GOST "GSI. Standards of units of physical quantities. Procedure for development and approval, registration, storage and application.” Standards are classified according to the principle of subordination. For this parameter, standards are primary and secondary.
The secondary standard reproduces the unit under special conditions, replacing the primary standard under these conditions. It is created and approved to ensure minimal wear and tear on the state standard. Secondary standards can be divided according to purpose. So, they distinguish:
1) copy standards, designed to transfer unit sizes to working standards;
2) comparison standards, intended to verify the integrity of the state standard, as well as for the purpose of replacing it if it is damaged or lost;
3) standards-witnesses, intended for the identification of standards, which for a number of different reasons are not subject to direct comparison with each other;
4) working standards, which reproduce the unit from secondary standards and serve to transfer the size to a standard of a lower rank. Secondary standards are created, approved, stored and used by ministries and departments. \
There is also the concept of “unit standard”, which means one means or a set of measuring instruments aimed at reproducing and storing a unit for subsequent transmission of its size to lower measuring instruments, made according to a special specification and officially approved in the prescribed manner as a standard. There are two ways to reproduce units based on technical and economic requirements:
1) centralized method - using a single state standard for an entire country or a group of countries. All basic units and most of the derivatives are reproduced centrally;
2) decentralized reproduction method - applicable to derived units, information about the size of which is not transmitted by direct comparison with the standard.
There is also the concept of “standard measuring instruments”, which are used for the regular translation of unit sizes in the process of checking measuring instruments and are used only in departments of the metrological service. The category of a standard measuring instrument is determined during metrological certification measurements by one of the bodies of the State Committee for Standards.
The quality of measurements is understood as a set of properties that determine the receipt of results with the required accuracy characteristics and in the required form.
The quality of measurements is characterized by such indicators as accuracy, correctness, reliability, convergence and reproducibility of results.
Measurement accuracy– quality of measurement, reflecting the closeness of its result to the true value of the measured quantity. Quantitatively, accuracy can be expressed by the reciprocal of the relative error taken modulo.
Correct measurements– this is a characteristic of the quality of measurements, reflecting the closeness to zero of the systematic error of the measurement results.
Reliability of measurements is determined by the degree of confidence in the measurement result and is characterized by the probability that the true value of the measured quantity is within the specified limits.
Convergence of measurement results– characteristic of the quality of measurements, reflecting the closeness to each other of the results of measurements of the same quantity, performed repeatedly using the same methods and measuring instruments and under the same conditions.
Reproducibility measurement results - a characteristic of the quality of measurements, reflecting the closeness to each other of the measurement results of the same quantity, obtained in different places, by different methods and measuring instruments, by different operators, but reduced to the same conditions.
Classification of measurements
Measurements are classified according to several criteria.
A) According to the dependence of the measured value on time:
static(the measured value remains constant over time during the measurement process);
dynamic(the measured value changes during the measurement process).
b) According to the existing sets of measured quantities:
electric;
mechanical;
thermotechnical;
physico-chemical;
radiation;
etc.
c) According to the conditions determining the accuracy of the result:
measurements of the highest possible accuracy, achievable with the current level of technology. These are measurements related to the creation and reproduction of standards, as well as measurements of universal physical constants;
control and verification measurements, the errors of which should not exceed a given value. Such measurements are carried out by state and departmental metrological services;
technical measurements, in which the error of the result is determined by the characteristics of the measuring instruments. Technical measurements are the most common and are performed in all sectors of the economy and science. These include, in particular, technological measurements.
d) By the number of measurements (observations) performed to obtain the result:
measurements with a single observation ( ordinary);
measurements with multiple observations ( statistical).
In this case, observation during measurement means an experimental operation performed during the measurement process, as a result of which one value is obtained from a group of value values that are subject to joint processing to obtain measurement results.
e) By the method of obtaining the result (by the type of measurement equation):
direct measurements– measurements in which the desired value of a quantity is found directly from experimental data. In the process of direct measurement, the measurement object is brought into interaction with the measuring instrument and, according to the latter’s readings, the value of the measured value is counted or the specified measurements are multiplied by a constant coefficient to determine the value of the measured value. Mathematically, a direct measurement can be described by expression (2). Example direct measurements can be: measuring length with a ruler, mass with a scale, temperature with a thermometer, etc. Direct measurements include measurements of the vast majority of parameters of chemical technological processes.
indirect measurements- measurements in which the desired value of a quantity is found on the basis of a known relationship between this quantity and the quantities subjected to direct measurements.
Example indirect measurements can be measurements of: the density of a homogeneous body by its mass and volume, electrical resistance by voltage drop and current strength, etc.
In modern microprocessor-based measuring instruments, calculations of the desired measured value are very often carried out “inside” the device. Measurements carried out by this kind of measuring instruments are referred to as direct measurements. Indirect measurements include only those measurements in which the calculation is carried out manually or automatically, but after receiving the results of direct measurements. In this case, the calculation error can be taken into account separately.
aggregate measurements– measurements of several quantities of the same name carried out simultaneously, in which the desired values of the quantity are found by solving a system of equations obtained by direct measurements of various combinations of these quantities.
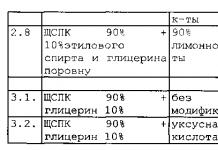
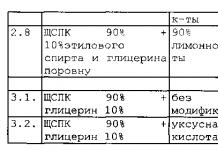
Example. Finding the resistances of two resistors based on the results of measuring their resistances when the resistors are connected in series and in parallel.
R2= (R 1 *R 2)/ (R 1 +R 2)
joint measurements– simultaneous measurements of two or several non-identical quantities to find the relationship between them.
For example. When determining the dependence of the resistor resistance on temperature, use the well-known expression:
where R t is the resistance of the resistor at a certain temperature t; R 20 – resistor resistance at a temperature of 20 o C; α and β are temperature coefficients. The required values of R 20 , α and β are found by solving a system of three equations compiled for three different temperature values. Here, resistance R t and temperature t are measured in a direct way.
In addition to the above criteria for classifying measurements for specific cases, others can be used if necessary. For example, measurements can be divided depending on the place of execution into laboratory and industrial; depending on the execution procedure over time - continuous and periodic; depending on the form of presentation of the results - absolute and relative, etc.